Introduction: The introduction of PET/CT in the staging and response assessment to therapy has changed the clinical evaluation of most lymphomas, especially Hodgkin lymphoma and aggressive B-cell lymphomas. The use of PET/CT in the staging of peripheral T-cell lymphomas (PTCL) is already a standard of care in many centers, although its decisional role in course of chemotherapy or at the end of treatment remains unclear.
Objective: The aim of this study is to evaluate the predictive role of interim PET/CT in newly diagnosed PTCL patients.
Methods: We retrospectively analyzed the clinical data of adult patients diagnosed with PTCL and actively treated between 2014 and 2023 at our center. All patients were evaluated with PET/CT at different timepoints (baseline, interim and end of treatment).
Results: We evaluated 49 patients, with a median age at diagnosis of 59 years (range: 18-83), and a slight predominance of females (54%) (table 1). Almost half of the patients were diagnosed of angioimmunoblastic/T-follicular helper lymphoma (AITL/TFHL) 23(47%), followed by anaplastic large cell lymphoma (ALCL) in 17 cases [9 ALK+ (18%) and 8 ALK- (17%))] and peripheral T-cell lymphoma NOS (PTCL-NOS) in 9 (18%). Seven (14%) patients had ECOG ≥ 2, 47 (90%) had stage III-IV disease, among them, 42 (84%) with extranodal involvement. Bone marrow was involved in 14 out of 36 (39%) evaluated patients. Twenty-seven (54%) patients had IPI score 3-5. Front-line treatment consisted in CHOP in 32 (65%) patients and brentuximab vedotin+CHP in 17 (35%). Interim evaluation was performed after 3 cycles in 32 (65%) and after 4 cycles in 16 (33%) patients. Only one patient was evaluated after 2 cycles due to suspected disease progression. At this time point, 31 (63%) patients were considered in complete metabolic response (CMR) with a Deauville Score (DS) 1-3, 12 (25%) in partial metabolic response (PMR) with a DS 4 and 6 (12%) patients with progressive disease (PD) with DS5. Among the latter's, 4 patients were treated with 2 nd line treatment, while 2 completed the planed 6 cycles due to significant tumor burden . Among 48 patients evaluated after 3 and 4 cycles of therapy, CMR was achieved in 18 (56%) and 13 (81%), respectively (p=0,088). Eleven (34%) and one (6%) pts were in PMR at iPET after 3 and 4 cycles, respectively.
Forty-five (92%) patients completed front-line therapy and were evaluated with EOT PET/. CMR (DS1-3) was reported in 32 (71%) patients, PMR in 8 (18%) and PD in 5 (%). Among patients with CMR at iPET, 25 (81%) maintained CMR, 5 (16%) converted to PMR and only 1 (3%) progressed. Among patients with PMR at iPET, 5 (42%) converted to CMR, 4 (33%) maintained PMR and 3 (25%) progressed. Eighteen (37%) patients were consolidated with autologous stem cell transplantation, all in CMR at EOT, except for 1 patient with PMR DS4.
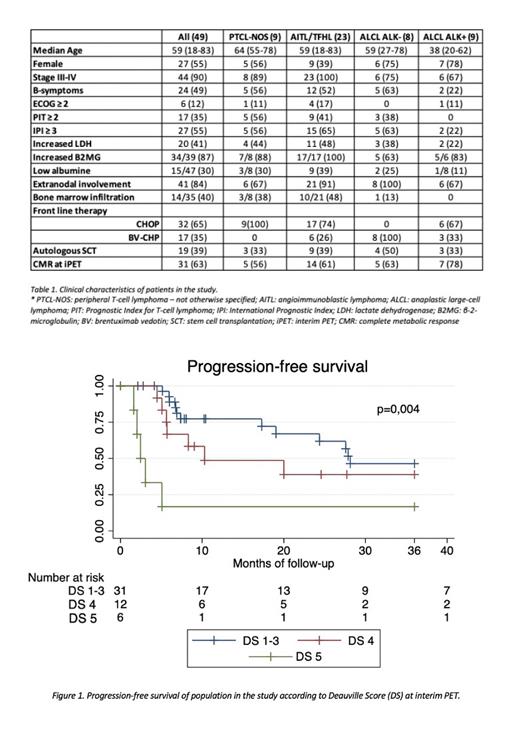
With a median follow-up of 28 months (range: 2-107 months), 25 (51%) patients relapsed or progressed. Median progression-free survival (PFS) of the entire population was 24 months. According to interim response, median PFS of DS1-3, DS4 and DS5 were 28, 10 and 2 months, respectively (Figure 1). In univariate analysis, along traditional prognostic factors as advance stage (p=0.0324), increased LDH (0.0211), IPI ≥ 3 (0.0198) and bone marrow infiltration (0.0475), interim PET/CT (iPET) impacted on PFS (DS1-3 vs DS4 vs DS5, p=0.004; DS1-3 vs DS4-5, p=0.0233). When considering the exact timing, iPET after 4 cycles seemed to relate better with PFS (DS1-3 vs DS4 vs DS5, p=0.0009) than after 3 cycles (DS1-3 vs DS4 vs DS5, p=0.32).
In the multivariate analysis, iPET/CT response maintained its prognostic significance (for DS1-3 vs DS4 vs DS5: HR 2.37, 95% CI 1,44-3,89, p=0,).
Median OS was not reached. The estimated 2-year OS for the entire population was 75% (95% CI, 58-86). According to iPET, the estimated 2-year OS for DS1-3, DS4 and DS5 were 85% (60-95), 64% (30-85) and 63% (14-90), respectively. No impact of iPET was documented on .
Conclusion: Our study highlights the potential role of interim PET/CT in the management of PTCL. Reaching an early metabolic complete remission seems to be associated with longer PFS. Patients with a DS4 at interim evaluation remain challenging for clinicians, since also in our study half of them converted to CMR at the end of . Further radiomics analysis may help to better identify patients at higher risk of progression and offer a more tailored treatment.
Disclosures
De Oliveira:Janssen: Other: Travel Expenses; Janssen, Alexion: Consultancy. Gonzalez Barca:Janssen, Abbvie, Takeda, EUSAPharma, AstraZeneca, Lilly: Speakers Bureau; Janssen, Abbvie, Kiowa, EUSA Pharma, Beigene, Sobi: Consultancy; Janssen, Abbvie, AstraZeneca: Other: Travel. Sureda Balari:Takeda: Consultancy, Speakers Bureau; Kite: Consultancy, Speakers Bureau. Domingo Domenech:BeiGene: Consultancy; BMS: Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal